
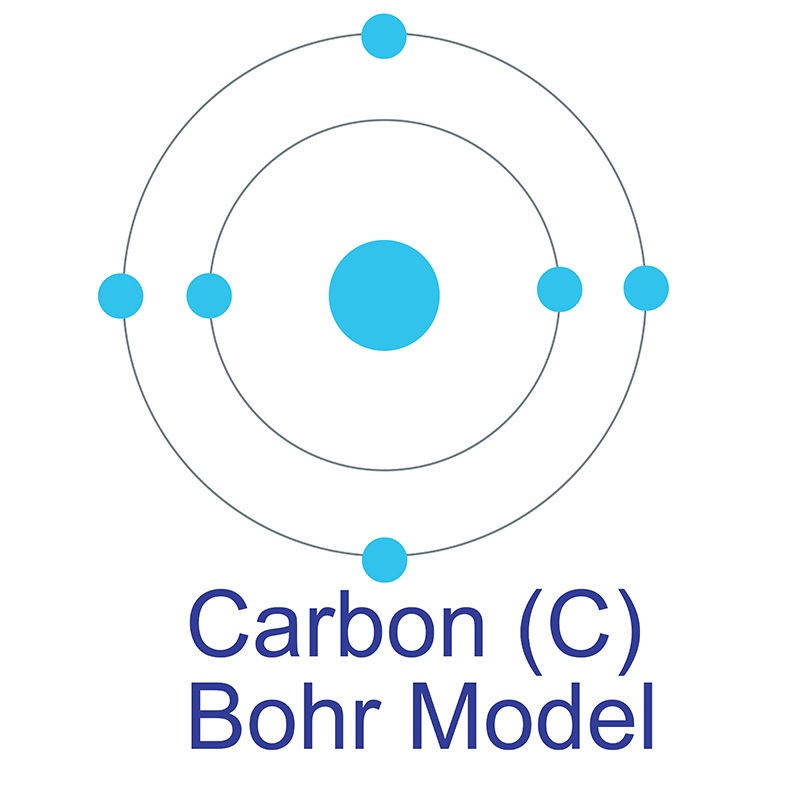
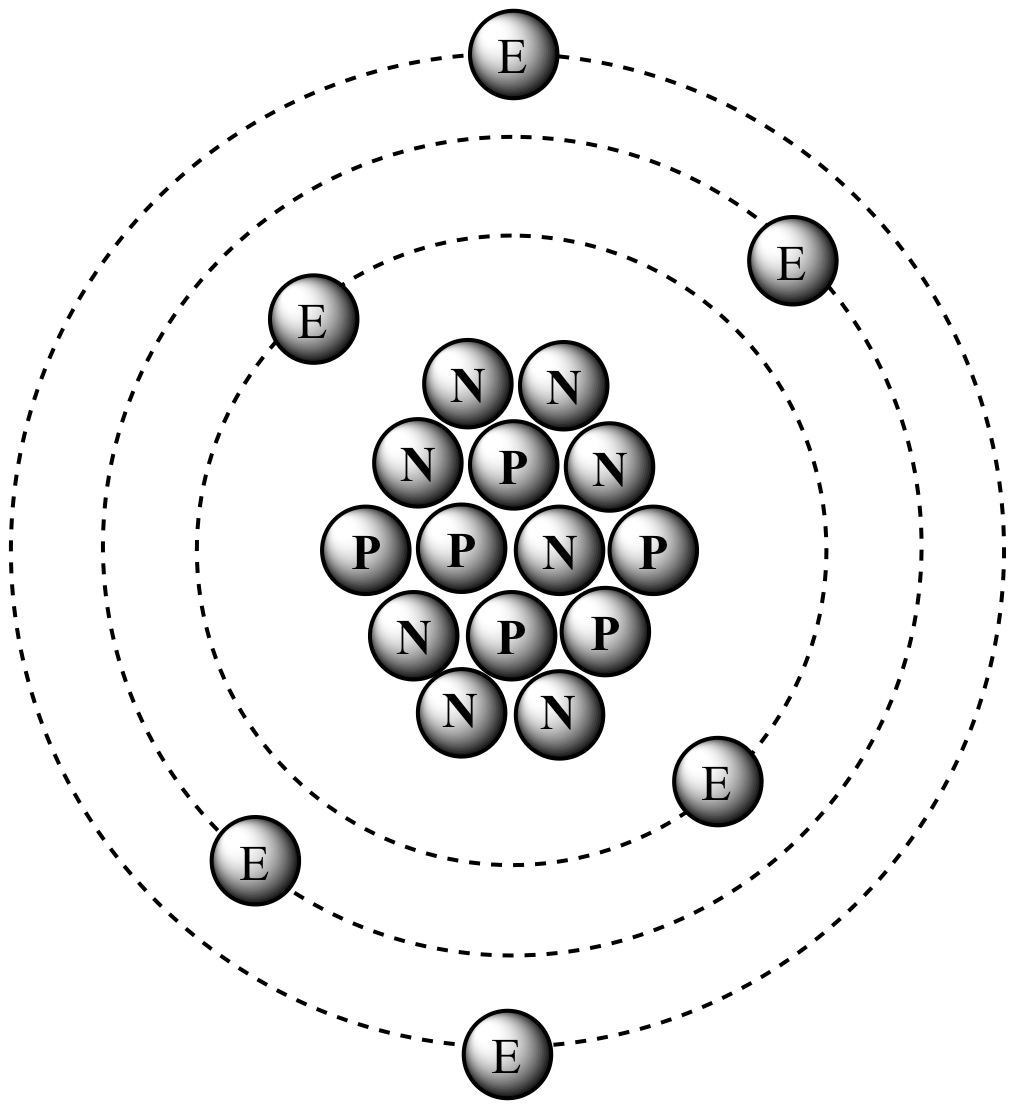
If all lower levels are filled an electron is at its ground state. They (electrons) can only drop to levels that are not filled. Think of the energy levels as a ladder, where the ground is the nucleus (gravity pulls everything to the ground, just like how electrons are pulled to the nucleus).Įlectrons increase their energy level by absorbing energy from an outside source ( heat, radiation, etc) which will cause them to move to higher up the ladder (exitied state).Įlectrons decrease to lower energy levels by rapidly giving off light which emit's energy. The bohr model describes most of the accepted features of the atomic theory without all of the high level math of the modern version.The Bohr model explains the Rydberg formula for the emission lines of atomic hydrogen, He tried to solve the question of why electrons do not stick to the nuclues. His work marked the first major step towards understanding where electrons are found in the atom. He also described the way atoms emit radiation by suggesting that when an electron jumps from an outer orbit to an inner orbit, it emits light. His model focussed on explaining the way electrons orbit the nucleus thrugh electron energy levels.īohr belived that there were definite orbits electrons could be in, the higher their energy the futher away they were.īohr was awarded a noble prize for his theory. He saw an analogy with the solar system, the nuc leus being the sun and electrons as planets.


 0 kommentar(er)
0 kommentar(er)
